Hospital Procedures
The basic steps involved in alcohol septal ablation are as follows:
- The procedure is performed in a cardiac catheterization laboratory.
- Patient is administered a sedative for relaxation of pain relief.
- The cardiac activity of the patient is monitored during and after the procedure through transesophageal echocardiography.
- Electrodes are placed on the chest to monitor the heart rate during the procedure.
- Tubes are inserted into the artery and vein in your groin and a temporary pacemaker is passed through the venous system to the right ventricle of the heart.
- A guide wire and balloon catheter are inserted through the tube and moved to your heart.
- Position of the septal artery is identified by employing a dye test, and then a balloon catheter is inserted to that region.
- The position of the balloon is confirmed by echocardiography and the balloon is inflated to temporarily block the septal artery.
- Alcohol (2-5 cc) is injected causing the muscles cells in that area to shrink or die.
- Finally the balloon is deflated and removed from the septal artery.
If there is a disturbance in the electrical activity of the heart, the temporary pacemaker is adjusted. If there is no such problem the temporary pacemaker is removed within 24 hours.
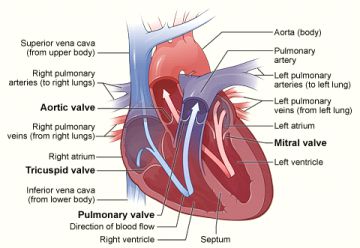
Heart valves that do not open fully (stenosis) can be repaired either with surgery or with a less invasive catheter procedure called balloon valvuloplasty. During the procedure, a balloon-tipped tube is threaded through your blood vessels and into the faulty valve in your heart. The balloon is inflated to help widen the opening of the valve. The balloon is then deflated and removed along with the tube. You are awake during this procedure which usually requires an overnight stay in the hospital. Balloon valvuloplasty relieves many of the symptoms of heart valve disease, but it may not cure it. The condition can still worsen over time. You may need medicines to help with the symptoms or surgery to repair or replace the faulty valve. Balloon valvuloplasty has a shorter recovery time than surgery. For some patients who have mitral valve stenosis, it may work as well as surgical repair or replacement. For these reasons, balloon valvuloplasty usually is preferred over surgical repair or replacement for these people.
During the procedure, a catheter is inserted into an artery in the arm or leg. Under x-ray guidance, the catheter is advanced into the chambers of the heart and the coronary arteries. X-ray dye is injected into the coronary arteries to determine whether there is a blockage and usually into the main pumping chamber of the heart to evaluate its function.
Coronary angioplasty, also called percutaneous coronary intervention (PCI), is a procedure used to open clogged heart arteries. Angioplasty involves temporarily inserting and inflating a tiny balloon within a blocked artery to help widen the artery.
Angioplasty is almost always combined with the permanent placement of a stent to help maintain long term patency of the diseased vessel. Some stents are coated with medication to help keep your artery open (drug-eluting stents), while others are not (bare-metal stents).
Angioplasty can improve some of the symptoms of blocked arteries, such as chest pain and shortness of breath. Angioplasty can also be used during a heart attack to quickly open a blocked artery, reduce the amount of damage to your heart and reduce the risk of death in some patients.
Angiography and Percutaneous Coronary Intervention (Stenting)
Left Heart Catheterization: Catheterization of the left side of the heart is performed via an atrial route. It is used to assess:
- Left ventricular function
- Outflow tract obstruction
- Valve disease
- Coronary artery disease
Cardiac Catheterization is also used to perform left ventricular biopsies and electrophysiological studies.
Right Heart Catheterization: Catheterization of the right side of the heart is performed through the venous route. It helps in the assessment of the following parameters:
- Measurement of cardiac output, left ventricular filling pressure and pulmonary artery wedge pressure
- Measurement of right heart oxygen saturations
- Assessment of pulmonary hypertension
- To perform an electrophysiological study.
Rotoblation: The use of a tiny drill, powered by compressed air, to remove calcified deposits. This is usually followed by balloon angioplasty, and the implantation of one or more stents.
CTO-PCI: Chronic Total occlusion-Percutaneous Coronary Intervention (CTO-PCI) is a procedure used to open blocked arteries. CTO is a serious condition in patients, and is very hard to treat. The occlusion prevents circulation to parts of the heart. The blockage is typically large and solid, having been present for three months or longer. CTO symptoms can include angina or pain in the chest, upper body and arms, jaw pain, indigestion or a choking feeling, cold sweat, and unusual fatigue or exercise intolerance. This procedure allows patients who historically have been unable to undergo coronary artery bypass surgery to have a less invasive approach to improve circulation.
PCI is performed by taking an antegrade (following the original course of the artery) or retrograde (going in the back door through side branches) approach. Essentially a catheter is inserted into the wall of the artery, going around the part of the artery that is completely blocked and then re-entering the artery to restore blood flow to the area that was not receiving proper circulation.
Cardiac resynchronization therapy (CRT) can relieve CHF symptoms by improving the timing of the heart’s contractions, or beats, which protects patients from abnormally slow and fast heart rhythms. CRT uses a biventricular pacemaker (or defibrillator) with two wires in the lower chambers of the heart to overcome this slow or abnormal conduction. By delivering simultaneous or near simultaneous electrical impulses to both lower heart chambers (the right and left ventricles), it causes the heart to beat in a more synchronized, efficient manner.
Cerebral angiography is a procedure that uses a special dye and X-rays to see how blood flows through the brain. Cerebral angiography is done in the hospital. An area of your body, usually the groin, is cleaned and numbed with a local numbing medicine. A thin, hollow tube called a catheter is placed through an artery and carefully moved up through the main blood vessels in the belly area and chest and into an artery in the next. X-rays help guide the doctor to the correct position. Once the catheter is in place, a special dye goes through the catheter. X-ray images are taken to see how the dye moves through the artery and blood vessels of the brain. The dye helps highlight any blockages in blood flow. After the X-rays are taken, the needle and catheter are withdrawn. Pressure is immediately applied on the left at the site of insertion to stop the bleeding. After that time, the area is checked and a tight bandage is applied.
Echocardiogram (TTE and TEE)
Cardiac CT angiogram (64 slice CT) with calcium scoring
An electrocardiogram (EKG) is a non-invasive, painless test that detects and records the electrical activity of the heart. EKGs are used to evaluate signs and symptoms that could indicate heart problems. It takes only a few minutes to perform.
Atrial fibrillation is a common disorder patients have that effects their heart rate or rhythm. It occurs when disorganized electrical signals cause the atria to contract very rapidly and irregularly. As a result, the blood is not pumped completely into the ventricles and collects in the atria. The atria and ventricles therefore do not contract in a coordinated manner resulting in either too much or not enough blood being sent from the heart to the body. Patients with atrial fibrillation may not have any symptoms, however, some people may experience chest pain and can cause heart failure and increased risk of stroke. Atrial fibrillation can be treated by medications and certain medical procedures to restore the heart’s rhythm. Blood thinners may be prescribed to reduce the risk of blood clots and stroke. Cardioversion is a procedure where electrical shock is delivered to your heart to restore its normal rhythm. If medications and cardioversion does not work, then ablation therapy is recommended. Ablation therapy creates scar tissue near the site of the origin of the abnormal electrical impulses to terminate them to prevent them from spreading. This can be done either nonsurgically or surgically depending on the type of arrhythmia and the presence of other cardiac problems.
Nonsurgical ablation
- A special catheter is inserted through veins in the groin and guided into the specific area of the heart to ablate the abnormal electrical signals.
Surgical Ablation
- This is frequently combined with other heart surgeries such as mitral valve repair and coronary heart surgery. It involves either of the following procedures:
- Maze procedure: Involves a traditional open heart surgery where small cuts are made in the left and right atrium to disrupt the conduction of abnormal impulses. The resulting scar tissue then blocks the abnormal impulses from spreading. This surgery is done through an open incision with the heart stopped and the patient on bypass machine.
- Mini Maze procedure: A minimally invasive approach to the Maze procedure in which surgery is done through smaller incisions. Endoscopes are utilized to view the surgical area. It is done on a beating heart and does not require the use of heart-lung machine and can be performed with the use of robotic technology. Using smaller incisions, the mini maze procedure minimizes trauma, and provides benefits of smaller scars, decreased risk of infection, less bleeding, less post-operative pain, faster recovery, shorter hospital stay and quicker return to daily normal activities. No use of heart-lung machine provides additional benefits that include reduced risk of stroke, reduced risk of renal failure and fewer cognitive side effects after surgery.
- Modified Maze procedure: A single incision in the left atrium is made to gain access to the heart to block the abnormal electrical impulses and to correct electrical conduction pathway.
- In cryotherapy, cold energy is also used for catheter-based ablation treatment of arrhythmia. It is particular useful for ablation around the atrioventricular node. During cryohterapy, the removal of heart from the tissue is performed rather than injected of cold energy into the tissue. This creates a temperature gradient ranging from sub-zero cryogenic temperature at the tip/tissue interface of the catheter to body temperature at sites away from the catheter tip. The cryo zone expands during freezing and shrinks upon rewarming. The cooling and freezing in the living tissue thus affects the cellular function and activity and causes ablation. Cryotherapy is quite safe and effective and is mainly used in peri-nodal procedures.
Biventricular pacemaker is a special pacemaker which is used to synchronize the contractions of the left ventricle with the right ventricle to improve the ejection fraction in patients with severe symptoms of heart failure. Ejection fraction (EF) is a measure of blood that is pumped out by the left ventricle of the heart and is expressed in percentage. A normal EF is usually between 50% to 70%. A patient with heart failure has a low EF hampering the blood supply to various parts of the body and leads to symptoms of heart failure that include shortness of breath, dry cough, swelling of the ankles and legs, weight gain, increased urination, fatigue, and rapid or irregular heartbeat. Biventricular implantation is considered in heart failure patients with:
- Severe or moderately severe heart failure symptom
- Delayed electrical activation of the heart
- Either a risk or histry of cardiac arrest
- On long-term medications for heart failure.
Similar to a standard pacemaker, a biventricular pacemaker can be implanted either by an endocardial (transvenous) approach or an epicardial approach. However, a biventricular pacemaker usually has three leads, one is guided to the right ventricle, one to the left ventricle and the third (which is not always present) is guided to the right atrium. The lead in the left ventricle is guided through the coronary sinus. When the heart rate drops below the rate set with the pacemaker, it senses the drop and transmits electrical impulses to the left as well as the right ventricle to contract simultaneously, improving the EF and the cardiac function. The lead placed in the right atrium helps the heart to function in a more balanced way. Biventricular pacemaker implantation is also called cardiac synchronization therapy and is only a part of any comprehensive heart failure management program. Medications, life style changes and regular follow up with a cardiac specialist are all crucial for managing the symptoms and improving the quality of life in heart failure patients.
An implantable cardioverter defibrillation (ICD) is a small electronic device that is implanted in the chest or abdomen to control abnormal heart rhythm and prevent sudden cardiac arrest. The ICD detects the abnormal heart rhythms and restores a normal heart rhythm by delivering electrical impulses to the heart muscle. Your doctor may recommend ICD if you have:
- Had a previous cardiac arrest or ventricular fibrillation or ventricular tachycardia
- Heart conditions such as dilated cardiomyopathy or hypertrophic cardiomyopathy that affect the heart muscle.
- An inherited condition such as long QT syndrome, Brigades syndrome and arrythmogenic right ventricular dysplasia
An ICD device contains one or more insulated wires called leads and a defibrillator unit, a small metal box that contains a pulse generator, computer and a battery. The device is connected to the heart muscle through leads with electrodes at their end. The lead thus detects the heart’s electrical activity and also delivers impulses from the device to the heart muscle. There are three different types of ICDs:
- Singe chamber ICD: These types of ICDs use a single lead which is placed in the right ventricle heart chamber. Energy is delivered to the right ventricle when required to help it contract normally.
- Dual Chamber ICD: In this type of ICD, the leads are attached in the right atrium and the right ventricle. These ICDs correct faulty electrical activity between an atrium and a ventricle. Energy is delivered to the right atrium first, and then to the right ventricle to restore a normal rhythm.
- Biventricular ICD: IN this typeof ICD, there are 3 leads which are placed in the right atrium, the right ventricle, and the left ventricle. This device helps the heartbeat in a more balanced way, and is specifically effective in patients with heart failure.
An ICD is usually implanted using the transvenous approach. In this approach, a small incision is made in the chest wall after numbing the area with local anesthetic. Sedation is also given to the patient. Through this incision, the lead is introduced into a vein to reach the specific chamber of the heart. This is done under guidance of real time X-ray images (fluoroscopy). The lead tip attaches to the heart muscle and the other end is connected to the pulse generator. Once the lead wires are in place, it will be tested for proper placement and functioning through a lead function test called pacing. The pulse generator is usually placed in a pocket created under the skin through the incision in the upper chest. The skin incision will be closed with sutures and a dressing will be applied once the procedure is complete. The whole implantation procedure usually takes between 2-4 hours. With the transvenous approach, patients recover faster and are discharged from the hospital in about 24 hours. If the transvenous approach cannot be used, the device is implanted in the pocket underneath the skin in the lower abdomen (epicardial approach). The epicardial approach is done under general anesthesia and requires a longer recovery time.
After the implantation procedure a chest X-ray is done to check the lungs and the position of the device and the leads. A telemetry monitor and holter monitor are also connected to the chest with sticky electrode patches to monitor and record the heart rhythm. These monitors are removed before the patient is discharged from the hospital. Then the patient is sent to the device clinic for final device settings. In the device clinic, a small device called a programmer is placed directly over the device to change the device settings and to check its function. AN echocardiogram may also be performed as part of your evaluation.
After implantation of an ICD, the patient should be scheduled for regular follow up visits. These follow up visits are important to monitor the function of the device and change the settings if required. Once implanted, an ICD usually lasts for 3-6 years before needing replacement.
A laser lead extraction is the laser technique employed to remove a pacemaker or defibrillator wire or wires from inside the heart. A cardiac pacemaker or an implantable cardioverter defibrillation (ICD) is implanted in cardiac patients to regulate heart rate or rhythm rhythm. Sometimes, due to an infected device pocket, or a broken or malfunctioning wire, these leads need to be removed.
Following device implantation, the leads of the device get encapsulated by the scar tissue and attach tightly to the vascular endothelium of the vein. The longer the device remains inside the body, the tighter the attachment, making removal difficult.
Laser assisted lead extraction is a safe and effective method to extract the leads in patients with chronically implanted cardiac pacemaker and defibrillator wires. This technique utilizes pulses of laser light to break the fibrous attachments without damaging other leads. During this procedure, an incision is made on the left side of the chest, usually close to the old device and the generator is removed and disconnected from the lead. For laser lead extraction, a special laser sheath is advanced through the targeted vein and over the lead. The sheath is connected to a laser that delivers energy to remove the scar tissue and detach the lead. The lead, laser and the sheath are removed at the end of the procedure. A new lead or a new pacemaker or ICD is then implanted immediately or at a later date. This technique uses cold, controlled laser energy which is absorbed only by the tissue lipids and proteins and not by water. It vaporizes the targeted sheath without damaging the surrounding lead.
Laser assisted lead extraction is thus a safer procedure with very few complications as compared to other extraction methods such as simple manual traction and use of locking stylets and outer sheath. It usually requires less time and is effective in most cases.
Tilt table procedure is a diagnostic procedure recommended to evaluate patients with unexplained fainting (syncope) and to confirm if the underlying cause is vasovagal syhndrome.
Vasovagal syndrome, also called neurogenic syncope, results from a dsyfunctioning of the nerves controlling the heart and the blood vessels. It leads to a sudden drop in blood pressure with or without a decrease in heart rate, causing episodes of fainting. These episodes usually occur while standing or sitting.
Tilt table procedure attempts to recreate similar situation of change in posture from lying to standing, by tilting the table from horizontal position to vertical position while the patient is safely strapped to the special table. The cardiovascular response is monitored by evaluating change in both ECG and blood pressure, especially during the position change of the table. If fainting occurs the table is immediately returned to the horizontal position. However, if no fainting occurs, the patient is administered a medication through an intravenous line to stimulate abnormal nervous system reflex that causes vasovagal syncope. The medication lowers the diastolic blood pressure (lower reading of blood pressure) and peripheral vascular resistance while increasing the heart rate. The table is again tilted to the upright position and cardiovascular response is monitored. Once adequate data is obtained, the bed is lowered and the patient is allowed to leave after some rest. A tilt table procedure is usually safe but may cause dizziness, headache, low blood pressure, high blood pressure, nausea, palpitation or change in heart rate which usually resolves once the table is returned to the horizontal position.
Holter and event monitors are medical devices that record the heart’s electrical activity and are used to diagnose arrhythmias.
Holter and event monitors also are used to detect silent myocardial ischemia. A Holter monitor is a portable device that is roughly the size of an iPod. It records all of your heartbeats on tape for a continuous period of 24-48 hours and is typically ordered when patients complain of symptoms like dizziness, syncope, or palpitations. A Holter monitor has electrodes that are attached to your chest with adhesive and then are connected to a recording device. Unlike Holter monitors, event monitors do not continuously record the heart’s electrical activity. They only record when symptoms occur. For many event monitors, you need to start the monitor when you feel symptoms. Information captured on the monitor’s recording device can be used to determine if you have a heart rhythm problem.
A mechanical assist device in the setting of acute myocardial infarction, high risk angioplasty, or in the setting of hemodynamic instability. This is a temporary assist device meant only to last a couple of days until transplant.
Inferior vena cava (IVC) filters are placed in patients who have a history of or are at risk of developing blood clots in the legs, including patients diagnosed with deep vein thrombosis (DVT), pulmonary embolus, trauma victims, immobile patients, recently had surgery or delivered a baby. IVC filters are used when patients cannot be successfully treated by other methods, including blood thinner agents.
Removal may be performed when the risk of clot traveling to the lung has passed. Removal of an IVC filter eliminates any long term risks of having the filter in place. It does not address the cause of deep vein thrombosis or coagulation. Your referring physician will determine if blood thinners are still necessary. However, not all retrievable IVC filters are able to be retrieved. These filters can be safely left in place as permanent filters.
IVUS: Intravascular Ultrasound (IVUS) is a test that uses sound waves to see inside blood vessels. It is useful for evaluating the coronary arteries that supply the heart.
Complete laboratory services and chest x-ray.
Ventricle Assist Device (VAD) is a mechanical pump that is implanted in the heart to help a weakened heart pump blood to the different parts of the body. These devices can be implanted in the right ventricle, left ventricle, or both ventricles of the heart. A VAD that supports only the left side of the heart is called a left ventricular assist device (LVAD). An LVAD, sometimes called a “bridge to transplant” can be surgically implanted to help improve the functioning of a weakened heart. These devices are available at heart transplant centers.
The LVAD can be used as a bridge-to-transplant, to support the weakened heart until a donor is available for a heart transplant. The LVAD can also be used as a destination therapy, to provide long-term support for patients with end-stage heart failure and are not eligible for transplant.
The most common type of LVAD possesses two tubes attached to a pump, which is placed in the upper part of the abdomen. One tube is inserted into the left ventricle and carries blood from the ventricle into the heart pump. The pump then transports the blood to the aorta, the main artery leaving the left ventricle. The pump’s other tube is guided through the wall of the abdomen and connected to the pump’s battery and control system, which are placed outside the body.
Modern LVADs are portable and are implanted for duration of a few weeks to months. Patients on LVAD support can have a satisfactory quality of life until a donor heart is available for transplant.
Studies have demonstrated that LVAD can normalize the heart function in a few patients with heart failure, obviating the need for transplantation.
A pacemaker is an artificial device for stimulating the heart muscle and regulating its contractions. The procedure to implant a pacemaker is usually quick. It does not require open-heart surgery, and most people go home within 24 hours. Before the surgery, medication is usually given to make you sleepy and comfortable. The procedure is performed under local anesthesia.
A small incision approximately 2 inches long will be made in the upper chest. One or two leads (thin insulated wires( will be guided through a vein into the heart. The doctor will then connect the lead(s) to your pacemaker and program the device for your medical needs. Then the pacemaker will be inserted beneath your skin, and the incision in your chest will be closed. Your doctor will test the pacemaker to ensure it is working properly to meet your medical needs
You will usually stay in the hospital overnight and go home the next day with instructions on caring for your incision. For a short time after surgery, your doctor may want you to limit how much you move the arm that is closest to your implant site. After the implant, there may be a slight bulge visible under the skin where the device is located. The leads are very thin and will not be visible.
An S-ICD is intended to provide defibrillation therapy for the treatment of life-threatening ventricular tachycarrhythmias in patients who do not have symptomatic bradycardia, continual ventricular tachycardia, or spontaneous frequently recurring ventricular tachycardia that is reliably terminated with anti-tachycardia pacing. It is implanted under the skin on the side of the chest below the arm pit. The pulse generator is connected to the electrode which is implanted under the skin from the device pocket along the rib margin to the breastbone with the use of the insertion tool.
The S-ICD monitors cardiac rhythms and delivers defibrillation when ventricular tachyarrhythmias are detected. After delivery of shock, the S-ICD provides post-shock bradycardia pacing therapy when needed. The S-ICD is programmable as a single or dual zone device which allows the doctor to tailor the therapy for the patient.
Although they are designed to be implanted permanently in the body, occasionally leads must be removed, or extracted. The most common reason for lead extraction is device infection. If any part of the system becomes infected, it is usually impossible to cure the infection without completely removing all hardware from the body. This requires removal of the pulse generator from the chest wall, as well as removal of all leads from the veins and heart. Another reason for lead extraction is when a lead fails to work properly (for example, due to a break in metal wire or surrounding insulation). Sometimes the broken lead can be abandoned in the heart, with a new lead placed alongside. However, veins can only accommodate a limited number of leads due to space constraints, and sometimes, nonfunctioning leads must be extracted to make space for a new lead. An uncommon reason for lead extraction is a mechanical lead failure that could be dangerous to the patient such as protruding wire.
Pacemaker and implantable cardioverter defibrillator leads are removed from the inside of the heart by use of specialized tools. The most common approach follows the course of the lead through the subclavian vein under the patient’s shoulder.
Patent Foramen Ovale (PFO) is a congenital heart defect, characterized by incomplete closure of the foramen ovale, an opening in the septum (wall) between the right and left upper chambers of the heart. The foramen ovale allows blood to bypass the fetal lungs. Normally the opening closes during infancy, but if it does not close it is called a patent foramen ovale. This type of defect generally works like a flap valve, opening up when the pressure inside the chest increases by coughing, sneezing or straining during a bowel movement. This pressure can open the defect, allowing blood to pass from the right atrium to the left atrium. If there is a clot in the blood, it can cross over to the left atria and can lodge in the brain causing a stroke or in the heart causing a heart attack.
A PFO usually causes no signs or symptoms and is not treated unless the child or adult has symptoms of transient ischemic attack (TIA) or stroke. A PFO can be detected with an echocardiography, chest X-ray and cardiac MRI scan. Blood thinning medications may be used for a patient with PFO who have had a stroke to reduce the risk of blood clot formation. Nonsurgical (catheter-based) closure of the hole is an alternative for individuals unable to take blood-thinning medications or those who have a second stroke while on medications.
The non-surgical closure of the PFO involves the following steps:
- A small puncture is made in your groin
- A thin, hollow, flexible tube (catheter) is inserted into a blood vessel in the groin and guided to the heart
- A balloon is placed and inflated across the opening to determine the size and location of the hold
- A PFO closure device is delivered to the heart through the catheter
- The device is positioned to close the hole with the imaging assistance of an echo transducer
- The device is then released from the catheter so that it covers each side of the defect and completely seals it closed
- The catheter is then removed and the closure device stays in place permanently in your heart
Atrial Septal Defect (ASD is a congenital heart defect, characterized by a hole in the muscular wall between the two upper chambers of the heart. During the development of the fetus, there is an opening between the upper chambers of the heart to allow blood to flow to the lungs. Normally the opening closes at the time of birth, butif it does not, the child is born with an atrial septal defect (ASD). The causes for the development are not clear. However, genetic and environmental factors may contribute to the development of the birth defect. Your doctor may assess an ASD through echocardiography, chest X-ray and MRI scan.
Treatment of ASD depends upon the type and size of the defect or presence of any other congenital heart defects. If an ASD is large, surgery to close the defect is recommended. ASD repair or closure may be performed through non-surgical methods or a surgical procedure. Depending upon your condition your surgeon determines the appropriate type of repair procedure.
The non-surgical closure of ASD involves the following steps:
- A tiny cut is made in the groin area
- A thing tube (catheter) is inserted into a blood vessel and guided to the heart
- As ASD closure device is attached to the catheter and advanced to the heart and through the defect under the guidance of X-ray and intra-cardiac echocardiogram
- The closure device is then placed across the ASD opening and the defect is closed. Eventually, heart tissue grows around the implant and becomes part of the heart tissue.
For high-risk patients and those who are not suitable for open-heart surgery, Transcatheter Aortic Valve Replacement (TAVR) may be an option. It is a less invasive procedure that does not require open-heart surgery. TAVR produces results in lengthening patients’ lives. TAVR allows the aortic valve to be replaced. This less invasive procedure allows a new valve to be inserted within the native, diseased aortic valve. The TAVR procedure can be performed through multiple approaches (i.e. transfemoral, transapical, or transaortic).
Transesophageal Echocardiogram (TEE) – is an endoscopic/ultrasound test that provides ultrasonic imaging of the heart from a retrocardiac vantage point, thus preventing the interposed subcutaneous tissue, bony thorax, and lungs from interfering with the ultrasound; it is performed to better visualize the mitral valve or atrial septum, to differentiate intracardiac from extracardiac masses and tumors, to diagnose thoracic aortic dissection, to detect valvular vegetation as seen with endocarditis, to determine cardiac sources of arterial embolism, to detect coronary artery disease, and to monitor high-risk patients for ischemia intraoperatively.